1. Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol 2006;20:981-996.

2. Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 2008;134:2101-2110.


4. Fu XB, Liu JY, Liu GN, Shao XM, Zhou XS. Computed tomography in predicting the efficacy of oral cholelitholysis with bile acids. Chin Med J (Engl) 1993;106:734-738.

5. Hickman MS, Schwesinger WH, Bova JD, Kurtin WE. Computed tomographic analysis of gallstones. An in vitro study. Arch Surg 1986;121:289-291.


6. Pereira SP, Veysey MJ, Kennedy C, Hussaini SH, Murphy GM, Dowling RH. Gallstone dissolution with oral bile acid therapy. Importance of pretreatment CT scanning and reasons for nonresponse. Dig Dis Sci 1997;42:1775-1782.

8. Polverosi R, Sbeghen R, Zambelli C, Caracciolo F, Spigariol F, Caroli A. [Role of computerized tomography in the densitometric assessment of lithiasis of the gallbladder]. Radiol Med 1992;84:387-392.

9. Tuncer I, Harman M, Mercan R, Ozturk M, Arslan I, Meral C, et al. The effects of ursodeoxycholic acid alone and ursodeoxycholic acid plus low-dose acetylsalicylic acid on radiolucent gallstones. Turk J Gastroenterol 2003;14:91-96.

10. Carrilho-Ribeiro L, Pinto-Correia A, Velosa J, Carneiro De Moura M. A ten-year prospective study on gallbladder stone recurrence after successful extracorporeal shock-wave lithotripsy. Scand J Gastroenterol 2006;41:338-342.


11. Rabenstein T, Radespiel-Troger M, Hopfner L, Benninger J, Farnbacher M, Greess H, et al. Ten years experience with piezoelectric extracorporeal shockwave lithotripsy of gallbladder stones. Eur J Gastroenterol Hepatol 2005;17:629-639.


12. Hellstern A, Leuschner U, Benjaminov A, Ackermann H, Heine T, Festi D, et al. Dissolution of gallbladder stones with methyl tert-butyl ether and stone recurrence: a European survey. Dig Dis Sci 1998;43:911-920.

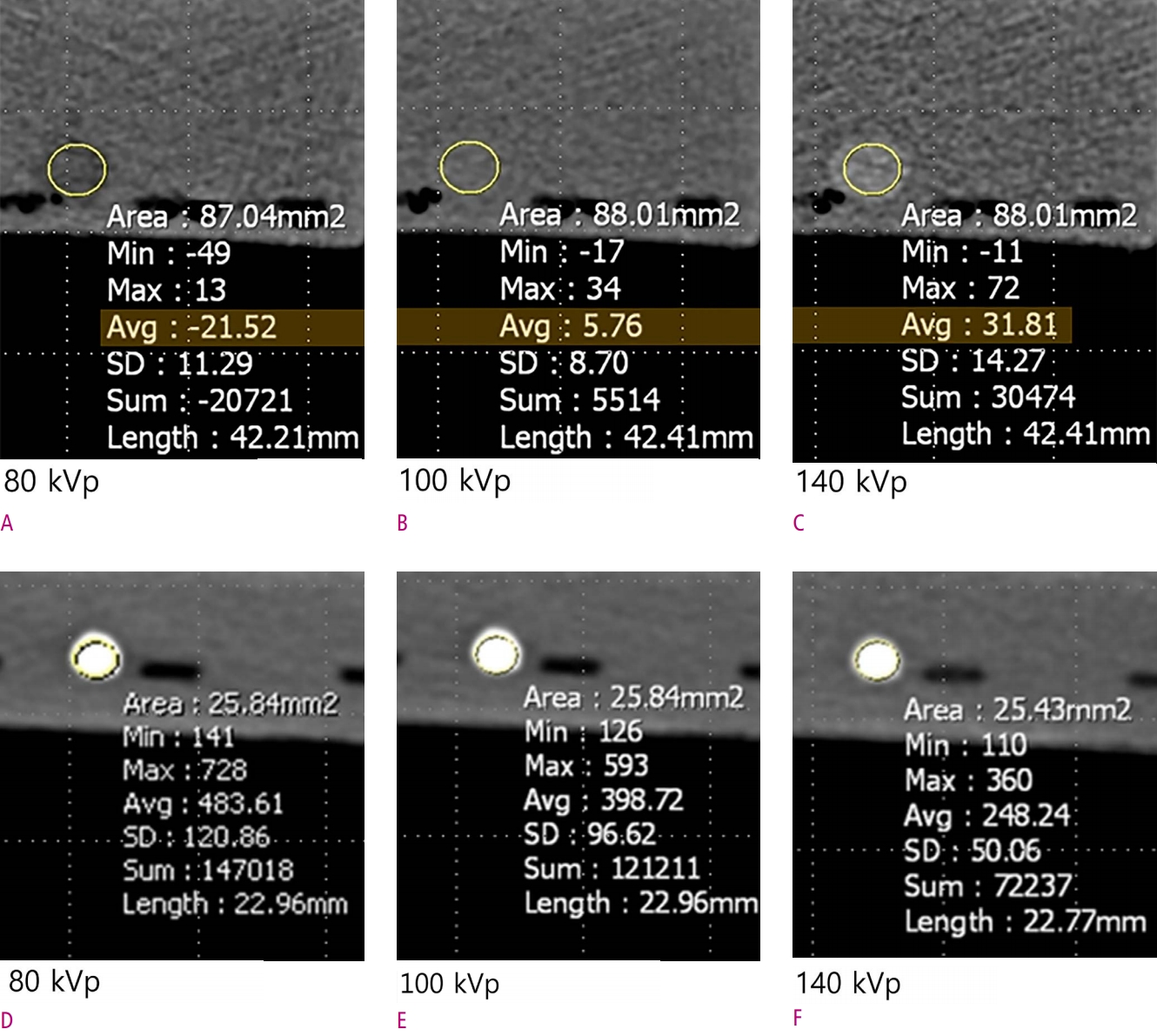
13. Zatz LM. The effect of the kVp level on EMI values. Selective imaging of various materials with different kVp settings. Radiology 1976;119:683-688.


15. Graser A, Johnson TR, Chandarana H, Macari M. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol 2009;19:13-23.


16. Fletcher JG, Takahashi N, Hartman R, Guimaraes L, Huprich JE, Hough DM, et al. Dual-energy and dual-source CT: is there a role in the abdomen and pelvis? Radiol Clin North Am 2009;47:41-57.


17. Yuh BI, Cohan RH. Different phases of renal enhancement: role in detecting and characterizing renal masses during helical CT. AJR Am J Roentgenol 1999;173:747-755.


18. Baron RL. Role of CT in characterizing gallstones: an unsettled issue. Radiology 1991;178:635-636.


19. Lee SP, Shuman WP, Teefey SA. CT evaluation of gallstones in vitro: correlation with chemical analysis. AJR Am J Roentgenol 1988;151:1123-1128.


20. Bensid A, Ucar Y, Bendeddouche B, Ozogul F. Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food Chem 2014;145C:681-686.

21. Bauer RW, Schulz JR, Zedler B, Graf TG, Vogl TJ. Compound analysis of gallstones using dual energy computed tomography--results in a phantom model. Eur J Radiol 2010;75:e74-80.


22. Coursey CA, Nelson RC, Boll DT, Paulson EK, Ho LM, Neville AM, et al. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics 2010;30:1037-1055.


23. Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol 2007;17:1510-1517.


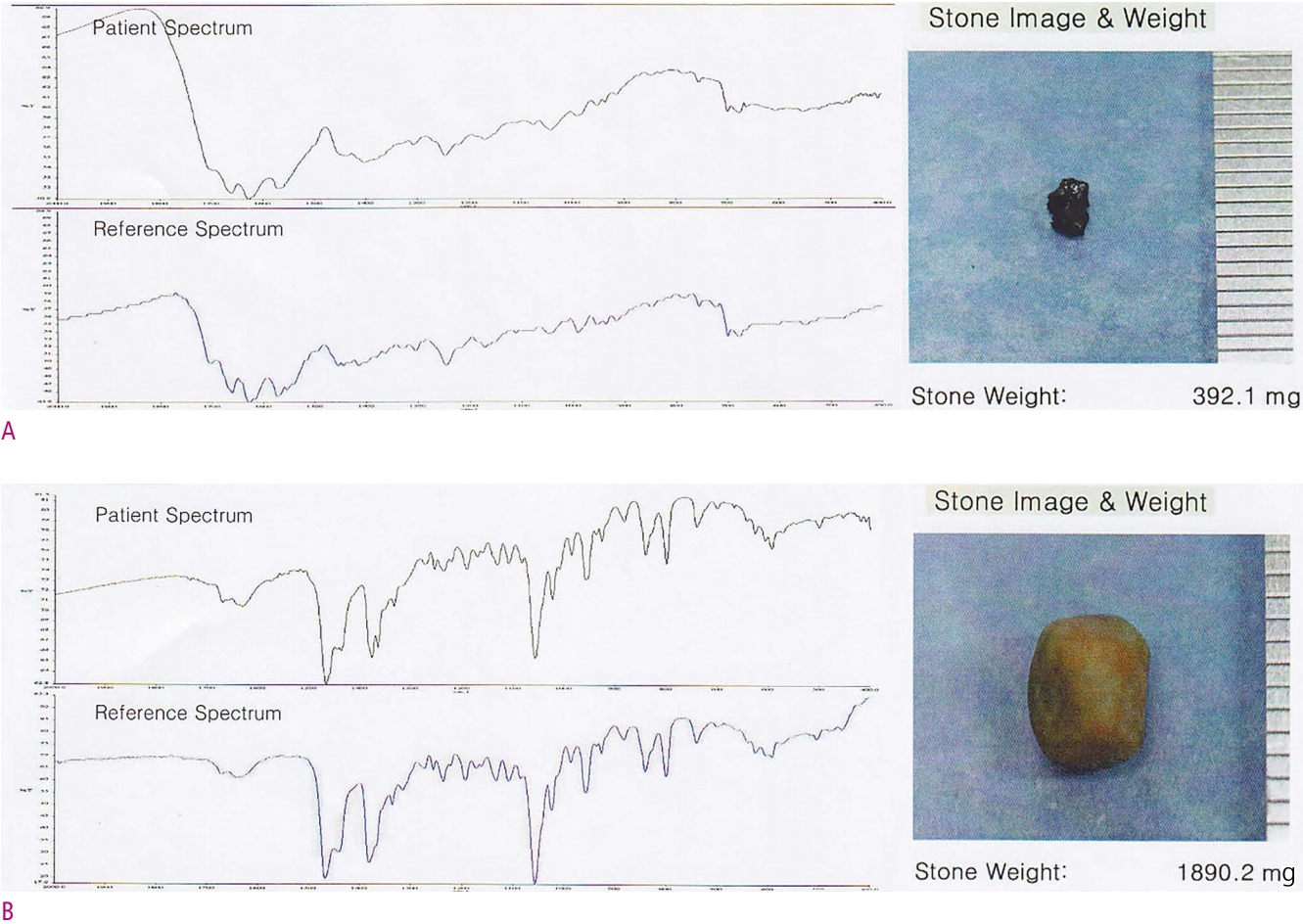
24. Berthomieu C, Hienerwadel R. Fourier transform infrared (FTIR) spectroscopy. Photosynth Res 2009;101:157-170.


25. Fuentes A, Fernandez-Segovia I, Serra JA, Barat JM. Effect of partial sodium replacement on physicochemical parameters of smoked sea bass during storage. Food Sci Technol Int 2012;18:207-217.


26. Vera Pingitore E, Bru E, Elena Nader-Macias MI. Effect of lyophilization and storage temperature on the activity of salivaricin CRL 1328, a potential bioactive ingredient of a urogenital probiotic product. J Gen Appl Microbiol 2012;58:71-81.


27. Schulte SJ, Baron RL. The effect of storage on the computed tomography attenuation of gallstones. Invest Radiol 1994;29:307-312.













 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print