Overview of Cholangiocarcinoma
On the basis of the anatomic location of origin, cholangiocarcinomas (CCA) are classified into intrahepatic CCAs (iCCA), perihilar CCAs (pCCA), and distal CCAs (dCCA). iCCA is defined as an adenocarcinoma located in the periphery of the segmental bile ducts (second-order bile ducts), pCCA involves the right and/or left hepatic ducts and/or their junction, and dCCA arises in the common bile duct [1]. The clinical significance of anatomic classification is that the tumor location implies the surgical extent. iCCA is typically treated through hepatectomy of the involved lobe or segments if resectable, while dCCA requires pancreaticoduodenectomy [1]. pCCA requires resection of the confluence of the bile duct, and can also include perihilar tumors with a significant intrahepatic component [1].
The incidence of iCCA is highest in Eastern countries such as Thailand, South Korea, and China, but its incidence and mortality are increasing worldwide [1]. Over recent decades, the worldwide age-standardized incidence for iCCA has been steadily increasing, whereas that for pCCA and dCCA has been decreasing [1, 2]. Several risk factors are known to be related to CCA. Although some are shared by all forms of CCA, others suggest greater specificity for one subtype and seem to be more important in different geographical regions [2]. A common characteristic among many of these risk factors is that they are associated with chronic inflammation of the biliary epithelium and bile stasis, such as choledochal cysts, biliary stones, primary sclerosing cholangitis, and liver flukes (Opisthorchis viverrini, Clonorchis sinensis) [2]. Several environmental toxins such as Thorotrast (banned in 1969), 1, 2-dichloropropane, asbestos, and tobacco smoking have been identified as risk factors for CCA. Several recognized risk factors such as viral infections (hepatitis B virus (HBV) and hepatitis C virus (HCV)), high alcohol consumption, obesity, metabolic syndrome, and nonalcoholic fatty liver disease have increased globally over recent decades and could be contributing to the increasing CCA rates [1-3]. However, in most locations, the majority of CCA cases remains sporadic without any identifiable risk factors [1].
Tumor Growth Pattern
The macroscopic growth pattern of CCA is somewhat different according to the anatomic location. iCCAs can exhibit three main patterns of growth: mass forming, periductal infiltrating, and intraductal growth [1, 4]. pCCAs and dCCAs present as peri-ductal infiltrating, nodular sclerosing tumors or, less frequently, as intraductal papillary tumors [4]. Morphologic classification is important to understand the mode of tumor spread, which is critical in predicting a tumor’s resectability and in planning the extent of surgery [5-7]. In regard to longitudinal extension, submucosal tumor spread is predominant in periductal infiltrating types, whereas mucosal spread is more common in intraductal growing or mass-forming types [7]. According to spread pattern, the length of discrepancy between microscopic and macroscopic tumor margins differs. In mucosal spreading, microscopic tumor spread beyond the macroscopic tumor margin has been reported to 10-20 mm, while 6-10 mm extensions have been observed submucosally [7]. Therefore, to obtain a negative surgical margin, it is recommended to resect at least 20 mm past the ends of the macroscopic tumor in intraductal growing or mass-forming types and at least 10 mm in periductal infiltrating types [7, 8]. Regarding transverse extension, periductal infiltrating type frequently extend beyond the bile duct wall and can directly involve adjacent organs or the hepatoduodenal ligament [7, 8]. This can result in perineural invasion, vascular invasion, and lymphatic spread, which suggest a poor prognosis as well as affect tumor resectability [7, 9]. Furthermore, different macroscopic growth types are associated with in different imaging findings and different lists of differential diagnoses.
This review provides a comprehensive and critical overview of the current literature for iCCA, focusing on mass-forming types.
Histological Subtype of iCCAs
Increasing evidence suggests that iCCAs are a heterogeneous group of tumors with varying etiologies, anatomic locations of origin, gross morphologies, histopathologies, and molecular features [1, 10]. Histologically, iCCAs can take the form of conventional, cholangiolocarcinoma, and rare variants [1, 11]. Conventional iCCAs can be further classified into two main histological subtypes, small and large bile duct types, according to the level or size of the affected duct [12, 13] (Table 1). Small bile duct iCCAs present as a small-sized tubular or acinar adenocarcinoma with nodular growth invading the liver parenchyma with minimal to no mucin production [12, 13]. Large bile duct iCCAs arise in large intrahepatic bile ducts and comprise mucin-producing columnar tumor cells arranged in a large duct or papillary architecture [12, 13]. Small duct iCCAs are associated with chronic liver diseases including viral hepatitis without known precursor lesions [1, 10, 12, 13]. In contrast, large-duct-type iCCAs are associated with chronic bile duct disease, including an intrahepatic stone or parasitic infection, and are thought to arise from precursor lesions including biliary intraepithelial lesions or intraductal papillary neoplasms of the bile duct; and progress to invasive intraductal neoplasms, periductal, or mass-forming types of tumors [10, 12-14].
Imaging Phenotype and Differential Diagnosis of iCCA
The imaging features of iCCAs are thought to reflect the pathologic subtype and gross morphology of the tumor (Table 1).
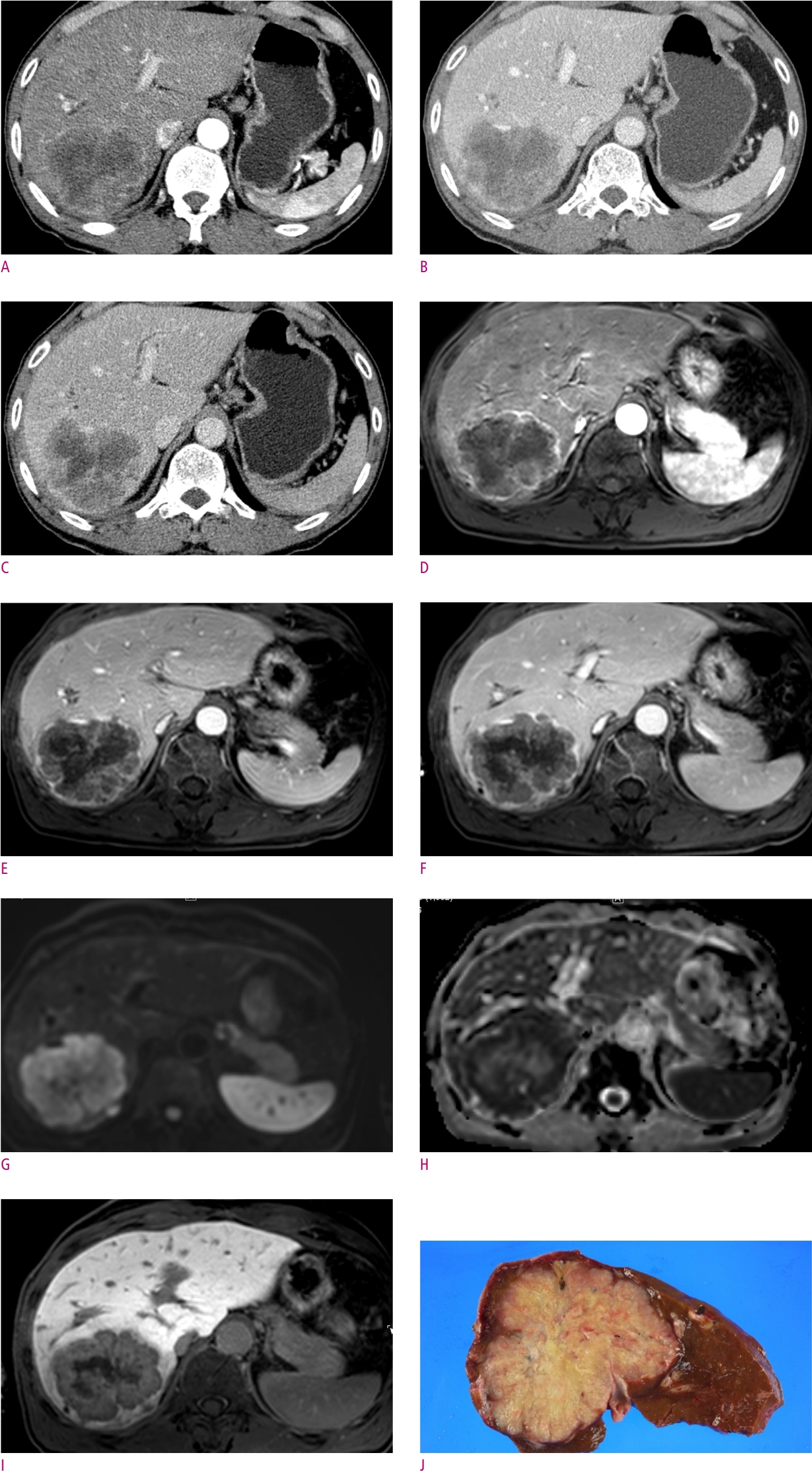
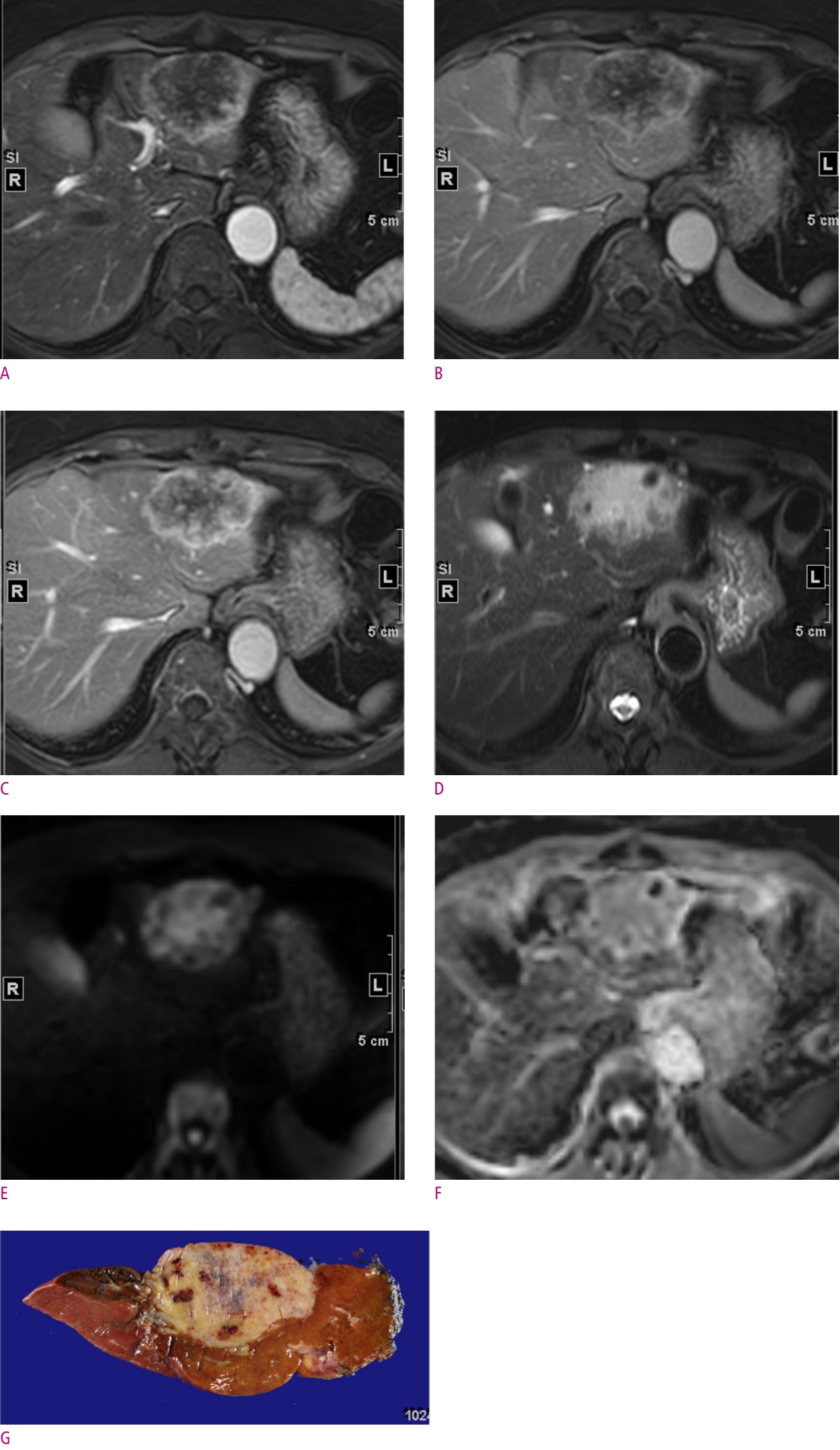
The typical imaging features of mass-forming iCCAs are a nonencapsulated mass of lobulated or irregular contour accompanied by hepatic capsular retraction and dilated peripheral bile ducts [7, 15]. On the arterial phase of dynamic contrast-enhanced CT and MRI images, iCCAs typically exhibit peripheral rim enhancement or diffuse hypoenhancement [6, 7, 15]. On portal venous or delayed phase, iCCAs present with peripheral washout or progressive centripetal enhancement [6, 7, 15]. Hypercellularity in the periphery of tumors with small extracellular volume contributes to peripheral enhancement in the arterial phase and peripheral washout appearance in the portal venous phase and/or delayed phase [7, 15]. On the other hand, central hypovascularity and mild progressive enhancement reflect the desmoplastic stroma with sparse tumor cells in the central portion of the tumor [7, 15]. The different compositions of the periphery and center of the tumor can present as a target-like appearance with peripheral hyperintensity and central hypointensity on high-b-value diffusion weighted images (DWI), reflecting the hypercellularity of the peripheral portion versus hypocellularity of the central portion with a fibrous component [16]. On the hepatobiliary phase (HBP) of gadoxetic acid-enhanced MRI, the reversed target-like appearance of peripheral hypointensity and central cloud-like hyperintensity can be of value in the diagnosis of iCCAs [7, 17] (Fig. 1).
Recently, atypical imaging features of mass-forming iCCAs have been reported more frequently, and several studies have suggested a subclassification based on imaging features [7, 10, 14, 18]. A recent single-center study divided mass-forming iCCAs into three groups according to arterial enhancement pattern on MRI - peripheral rim enhancement, diffuse hypoenhancement, or diffuse hyperenhancement [19]. Patients with diffuse hyperenhancement type on arterial phase exhibited more frequent chronic liver disease (13 of 20; 65%), less frequent vascular invasion (6 of 20; 30%), and less frequent tumor necrosis (3 of 20; 15%) compared to peripheral rim enhancement or diffuse hypoenhancement type (19) (Fig. 2). Another study recommended subdivision of mass-forming iCCAs into parenchymal and ductal types and revealed more frequent arterial hypervascularity and better overall survival in the parenchymal type [10, 19-21].
The diffuse arterial phase hyperenhancement pattern of iCCAs can mimic HCCs. Sharing risk factors of chronic liver disease makes it more difficult to differentiate between on through imaging. A case-control study to differentiate iCCAs from HCCs in patients with cirrhosis confirmed that the typical enhancement patterns of iCCAs - peripheral/rim enhancement in the AP followed by persistent or progressive contrast enhancement in the PVP and TP - are more frequent than HCCs in cirrhotic livers using gadoxetic acid-enhanced MRI, similar to extracellular contrast agent-enhanced CT and MRI in noncirrhotic livers [18-21]. In addition to the dynamic enhancement pattern, peritumoral bile duct dilatation and target appearance were independent and significant factors suggestive of iCCAs. A well-known pitfall of gadoxetic acid-enhanced MRI is the misinterpretation of atypical iCCA as HCC owing to the “pseudo washout” effect [7]. To avoid false diagnoses, an exclusive washout definition on PVP of gadoxetic acid-enhanced MRI was suggested but led to a significant decrease in sensitivity for HCCs [7, 21]. Considering the extremely lower prevalence of atypical (global wash in and washout) enhancing iCCAs compared to HCCs, however, it is difficult to accept the washout definition exclusively based on PVP in daily practice. Moreover, the prognosis following treatment with the same method used for equivalent-stage HCCs has not been well-established and has undefined clinical relevance [22-24].
Combined hepatocellular cholangiocarcinomas have been reported to be more commonly misdiagnosed as iCCAs rather than HCCs on preoperative imaging even though they can mimic either [25]. The presence of the target-like appearance on hepatobiliary phase images, the absence of major vascular thrombosis, and the presence of intrahepatic bile duct dilatation suggest iCCAs over combined hepatocellular cholangiocarcinomas. The preoperative differential diagnosis of iCCAs on the basis of imaging features typically is not possible, and biopsies should be considered for confirmation of the diagnosis [25]. Benign lesions mimicking iCCAs include liver abscess and sclerosing hemangioma (Fig. 3).
Staging of iCCAs
iCCAs, pCCAs, and dCCAs differ in tumor characteristics and have distinguishing American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) stage [1]. Before the 7th edition of the AJCC system in 2010, iCCAs shared the same stage as HCCs [26-29]. In the 7th edition, the size cut-off of 5 cm in multiple tumors was discarded, and periductal infiltrating tumor growth type was considered to indicate a poor prognosis, staged as T4. In the 8th edition of the AJCC system, several major changes were made to the T staging of iCCAs - the T1 stage (solitary tumor without vascular invasion) was divided into T1a and T1b stages using a size cut-off of 5 cm; T2a (solitary tumor with vascular invasion) and T2b (multiple tumors with or without vascular invasion) stages in the 7th edition were unified into the T2 stage; extrahepatic organ invasion replaced periductal invasion for determination of the T4 stage [26-29] (Table 2). Since surgical resection is the only potentially curative treatment for patients with cholangiocarcinoma, it is important to accurately determine its clinical stage using imaging studies to identify surgical candidates. A recent multicenter cohort study showed that MRI exhibited superior sensitivity to CT for diagnosing tumor multiplicity, particularly gadoxetic acid-enhanced MRI [28]. Recent studies have demonstrated that intrahepatic metastasis is an important prognostic factor for iCCAs, and T2 tumors with multiplicity showed a worse prognosis than T3 tumors in the AJCC 8th edition, with one study even classifying them into the M1a stage [29]. It is critical to detect intrahepatic metastasis in iCCA, and the use of gadoxetic acid-enhanced MRI is recommended in addition to CT before surgery to detect additional intrahepatic metastases [28].
Preoperative Prognostic Prediction of iCCA
As mentioned earlier, several imaging features with better or worse prognosis have been reported based on tumor heterogeneity of iCCAs. Diffuse or nodular arterial phase hyperenhancement is the representative imaging feature correlated with better overall and/or progression-free survival after surgical resection [19]. Histologically, the area of hyperenhancement is correlated with the hypercellular component, whereas areas of delayed phase enhancement correspond to the fibrosis component [7, 19]. A recent study correlated enhancement pattern on CT with the subtype of conventional iCCA as small and large duct types, which exhibit different tumor aggressiveness and clinical outcomes [30]. They revealed that arterial hyperenhancement or rim enhancement was common in the small duct type, while arterial hypoenhancement was common in the large duct type [30]. A multicenter retrospective study suggested the use of preoperative MRI prognostic models that include serum CA19-9 and four MRI features (bile duct invasion, tumor multiplicity, lymph node metastasis, and cirrhosis) for calculating the overall survival of mass-forming iCCAs [31]. The preoperative MRI prognostic score showed comparable discriminatory performance to pathologic staging systems and might be used to determine an optimal treatment strategy [31].
Conclusions
iCCAs are highly aggressive and heterogeneous tumors with a poor prognosis. Tumor resection remains the only potentially curative option for these patients, and preoperative imaging diagnosis, resectability assessment, and accurate tumor staging are of great importance. Current concepts regarding risk factors, tumor biology, and histologic classifications might also allow for a better understanding of the diverse imaging features of iCCAs. Therefore, knowledge of the updated classification systems and current principles for management of iCCAs might be helpful in determining a clinically relevant interpretation of the imaging studies. Despite the recent advanced knowledge of iCCAs, it remains an open field of research with important gaps that need to be filled. Therefore, all efforts must be gathered to ultimately decipher the complexity of iCCAs.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print