완치 목적의 perihilar cholangiocarcinoma 수술 전 불완전 절제 및 불량한 예후 예측을 위한 CT 소견
Important CT Findings for Prediction of Incomplete (R1 or R2) Resection and Poor Survival of Perihilar Cholangiocarcinoma after Curative-Intent Surgery
Article information
Abstract
목 적
Perihilar cholangiocarcinoma의 불완전 절제 (R1 혹은 R2) 및 불량한 예후 예측을 위한 수술 전 CT 영상 소견을 평가하고자 한다.
방 법
2006에서 2012년까지 완치 목적의 수술을 받은 총 139명의 perihilar cholangiocarcinoma 환자의 수술 전 다중 CT 영상을 분석하였다. 두 명의 영상의학의사가 췌장 내 담도에서 담도 2차 분지지점까지 각 구간 별 종양의 침범 여부 및 병변 주변 지방 침범 가능성 (likelihood)를 5점 기준으로 평가하였고, 혈관 침범 (없음, 닿음, 둘러쌈), 및 림프절 침범 여부도 평가하였다. 각 영상 소견은 독립적으로 검토 후 합의 과정을 통해 최종 결정하였다. 수술 전 CT 상의 담도 침범 소견을 likelihood 점수 4점이상으로 하였을 때, 이 기준의 진단능을 병리 소견과 비교하여 receiver operating curve로 분석하였다. 절제 후 병리는 완전 절제 (R0)와 불완전 절제 (R+; R1 혹은 R2)로 분류하였다. 수술 전 CT 영상에서 불완전 절제의 예측 인자 확인을 위해 로지스틱 회귀분석이 사용되었다. 수술 전 CT 영상 및 혈액검사 소견을 통한 생존율 예측 인자 확인을 위해 Cox의 비례위험모형을 사용하였다.
결 과
71명의 환자는 완전 절제, 68명의 환자는 불완전 절제가 되었다. 절제 가능성 평가에서는, 다변수 로지스틱 회귀분석에서 수술 전 영상에서 총 담관 중간부 침범 (likelihood score ≥ 4)이 불완전 절제의 유의한 소견이었다 (p < 0.01). 다변수 Cox 비례위험모형 분석에서는, 췌장 내 담도 침범 (likelihood score ≥ 4, hazard ratio = 1.81, p < 0.01), 총 빌리루빈 상승 (hazard ratio = 1.53, p < 0.04), CA19-9 (hazard ratio = 1.75, p < 0.01)가 불량한 예후를 예측하는 유의미한 인자였다. 수술 전 CT를 이용한 총 담관 중간부 침범과 췌장 내 담도 침범 정확도는 각각 0.71과 0.72 (AUC 값) 이다.
결 론
수술 전 CT에서 perihilar cholangiocarcinoma의 원위부 침범 소견은 불완전 절제 및 불량한 예후를 예측하는데 유의미한 소견이다.
Trans Abstract
Purpose
To evaluate CT findings to predict incomplete (R1 or R2) resection and poor survival in patients with perihilar cholangiocarcinoma using pre-operative CT.
Materials and Methods
From 2006 to 2012, a total of 139 patients with perihilar cholangiocarcinoma who underwent pre-operative multiphase CT and subsequent curative-intent surgery were included. After two radiologists independently reviewed CT findings including the likelihood of bile duct (BD) involvement from intrapancreatic common bile duct (CBD) to bilateral second-order branches and peritumoral fat stranding using a 5-point scale, vessel involvement (no, abutment, encasement), and LN involvement, imaging findings were finalized by a consensus of two radiologists. When the likelihood scale of 4 or more on preoperative CT was regarded as BD involvement, the diagnostic ability of CT was analyzed by the receiver operating curve using histopathologic results as a reference standard. Residual tumor categorized into no residual tumor (R0) and residual tumor (R+; R1 or R2). Predictive factors of R+ resection on pre-operative CT were analyzed by logistic regression. Cox proportional hazard model was used to determine the prognostic factor for overall survival by using pre-operative CT findings and laboratory results.
Results
Seventy-one patients were R0 and sixty-eight patients were R+ resection. For resectability evaluation, mid-CBD involvement (score ≥ 4) in pre-operative CT was significant factor for R+ resection in multivariable analysis (P < 0.01) with substantial interobserver agreement. In multivariable Cox regression, intrapancreatic CBD involvement (score ≥ 4, hazard ratio [HR] = 1.81, P < 0.01) as well as elevated total bilirubin (HR = 1.53, P = 0.04) and CA 19-9 level (HR = 1.75, P < 0.01) were significant predictors for poor survival. Diagnostic ability to predict mid-CBD and intrapancreatic CBD involvement on pre-operative CT were 0.71 and 0.72 (AUC values).
Conclusions
Distal longitudinal extent of perihilar cancer on pre-operative CT is a significant factor for margin positive resection and poor survival on curative-intent surgery.
Introduction
Perihilar cholangiocarcinoma (PHC) is the most common malignancy of the bile duct, accounting for more than half of all cholangiocarcinoma [1]. Prognosis of PHC is grim with a median survival of 6 months without any treatment [2]. At present, complete surgical resection offers only a chance for cure and long term survival with a reported median survival of 19 to 39 months [3] in patients without distant metastasis. Many studies have been identified poor prognostic factors after curative-intent surgery of PHC, such as a positive resection margin, moderate to poor tumor differentiation, and perineural invasion [4-6]. Among them, tumor-free surgical margin is the most important and well known prognostic factor for PHC [6, 7]. However, tumor-positive surgical margin is frequently observed in resected PHC due to its anatomic location, which can spread longitudinally (intrahepatic or intrapancreatic bile duct) and/or transversely (hepatic artery, portal vein, perineural tissue or lymphatics) [8, 9]. Thus, many surgeons reported that extended surgery based on meticulous pre-operative evaluation is warranted to avoid incomplete resection, which undoubtedly leads to poor overall survival [10-13].
To determine resectability, contralateral hepatic artery or portal vein invasion, main portal vein invasion longer than 2 cm, small remnant liver volume, biliary extension to the contralateral secondary confluence with farther than 2cm from hepatic hilum on pre-operative cross-sectional images are the well known predictable factors for unresectability [14, 15]. However, even with meticulous pre-operative evaluation, there is still considerable margin positive resection on curative intend surgery [8, 9]. Recent advances in CT technology, such as multidetector CT (MDCT) and iterative reconstruction, enabled to achieve improved spatial and temporal resolution [16-18]. Indeed, recent studies have reported the substantial predictability of MDCT to determine resectability for gallbladder cancer [19] or pancreas cancer [20].
Herein, with a propounding development of CT and image reconstruction technology, we surmised that predicting resectability of PHC using pre-operative CT has become more accurate, and also it offers more information regarding resectability assessment and survival. Therefore, the purpose of our study is to reveal CT findings to predict margin positive resection (R1 or R2) and poor survival in patients who underwent curative-intent surgery of perihilar cholangiocarcinoma using pre-operative CT.
Materials and Methods
This study was approved by our institutional review board, and the requirement for informed consent was waived.
Patients
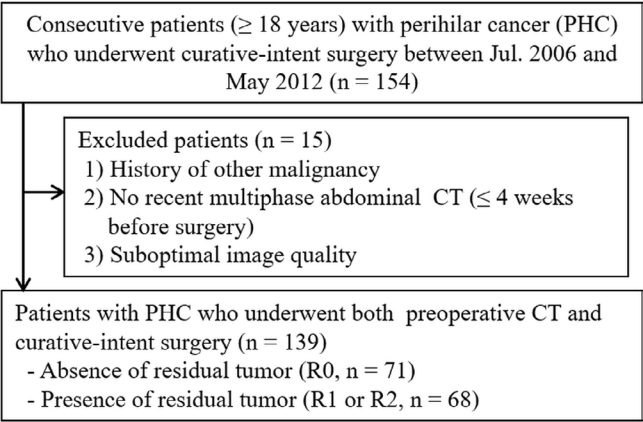
From 2006 to 2012, we searched electronic medical record in our hospital consecutively to identify eligible patients who met following criteria: i) adult patient (≥ 18 years), ii) pathologically confirmed perihilar cholangiocarcinoma, iii) who underwent curative-intent surgery and iv) pre-operative multiphase abdominal CT. Exclusion criteria were as follows: history of other malignancy including perihilar or elsewhere, no recent CT within 4 weeks before surgery, or suboptimal CT image quality. According to the criteria, a total of 139 patients (82 men and 57 women; mean age, 66.9 years, age range, 42-75 years) were selected for this study (Fig. 1). All 139 patients underwent curative-intent surgery by one of two pancreatobiliary surgeons with 27 and 18 years of experience in pancreatobiliary surgery in the same institute. The extent of surgery were preoperatively discussed with board certified surgeons, radiologists and internists in a multidisciplinary team meeting The mean interval (± standard deviation) between last CT examination and surgery was 17.5 days ± 11.0 (range, 0 to 28 days). Patients underwent extended right hepatectomy (n = 48), extended right hepatectomy with pancreaticoduodenectomy (n = 2), extended left hepatectomy (n = 26), extended left hepatectomy with pancreaticoduodenectomy (n = 1), hilar resection (n = 56), hilar resection with pancreaticoduodenectomy (n = 2), bypass or open and closure (n = 4). Detailed patient demographics are summarized in Table 1.
CT technique
All patients underwent four-phase multidetector CT (MDCT) (n=96) consisting of pre-contrast, early arterial, late arterial and venous phase, or three-phase MDCT (n=43) consisting of pre-contrast, arterial and venous phase before curative-intent surgery. Owing to retrospective nature of this study, various CT scanner were used. Detail parameters of each CT scanner were noted in Appendix 1. Contrast media was injected at a rate of 2.0 to 3.0 mL/sec using a power injector with a total volume of 1.5 mL per kilogram of the patient body weight followed by flushing with 20 mL normal saline. After contrast media injection, an automatic bolus tracking technique was used for image acquisition. The trigger threshold was 100 HU at the abdominal aorta. Early arterial phase was obtained 6 seconds after the trigger threshold and pancreatic phase was obtained 5 to 9 seconds after the early arterial phase. Venous phase was obtained 40 to 50 seconds after early arterial phase.
Image analysis
Two board-certified radiologists (I.J. and W.C, with 12- and 9-years of experience in abdominal imaging) independently reviewed all pre-operative MDCT images. They were blinded to patients' histological or operative results, but were aware that this study population had PHC. Reviewers were requested to determine the likelihood of tumor involvement of bile duct (BD) in longitudinal and transverse plane. For longitudinal plane assessment, the likelihood of tumor involvement from intrapancreatic common bile duct (CBD) to bilateral second order branches of intrahepatic bile duct (IHD) using 5-point scale as follows: score 1, definitely no, no significant image findings; 2, probably no, presenting only short stricture with smooth transition; 3, indeterminate, one positive image finding suggestive of malignancy; 4, probably yes, two positive image findings suggestive of malignancy; 5, definitely yes, more than three positive image suggestive of malignancy. The positive image findings suggestive of malignancy are long-segmental involvement (> 12 mm), a thickened (> 1.5 mm) wall, luminal irregularity or asymmetry, and incremental enhancement may favor a malignant stricture [21, 22]. For evaluating tumor involvement in the transverse plane to BD, following 5-point scale was used: score 1, definitely no, no significant image findings; 2, probably no, minimally increased attenuation in peritumoral fat; 3, indeterminate, increased attenuation with ground-glass like pattern in peritumoral fat; 4, probably yes, increased attenuation with reticular pattern in peritumoral fat; 5, definitely yes, obviously increased attenuation as much as BD wall [23]. The cutoff for tumor involvement was determined by cutoff values from the receiver operating curve analysis. Mid-CBD was defined as CBD below cystic duct or more than 2 cm away from 1st bifurcation of CBD.
Also, the relation between tumor and vessels (proper, left and right hepatic artery [PHA, LHA, and RHA]; main, left and right portal vein [MPV, LPV, RPV]) was evaluated using three categories: no relation, abutment (180 degrees or less of a vessel's circumference), encasement (more than 180 degrees of tumor involvement of a vessel's circumference). In artery evaluation, abutment or encasement classified as tumor involvement. While vein evaluation, encasement, change caliber of vessels in the region of contact, or irregularity of vessel margin were assessed as tumor involvement [24]. Furthermore, the anatomy type of BDs were assessed according to the five anatomy types reported by Huang et al. [25] namely, normal, trifurcation, right posterior BD draining to the left hepatic duct, right posterior BD draining to the common BD, and right posterior BD draining to the cystic duct. The conventional BD anatomy was defined as the right posterior BD draining to the right hepatic duct, with the right and left hepatic ducts joining the common hepatic duct. In addition, Bismuth type [26], and LN involvement (no significant LN enlargement, indeterminate LN, highly suspected metastatic LN) were also assessed. When LNs larger than 10 mm in short diameter, central necrosis or hyper-attenuating than liver in portal venous phase were considered highly suspected metastatic LN [14, 27]. Indeterminate LN was defined when only one positive findings of metastatic LN was noted.
Laboratory and histologic analysis
The pre-operative level of total bilirubin, albumin, alkaline phosphatase, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9) have been routinely checked for pre-operative planning of perihilar cancer in our hospital. When the patients received a biliary intervention to decompress the bile duct before surgery (n = 25, 18.0%), the laboratory results after biliary intervention were used for analysis.
For histologic analysis, all surgical specimens were reviewed by an experienced pathologist (K.L. with more than 17 years' experience in pancreaticobiliary pathology). Information of tumor location, tumor size, depth of invasion, lymph node involvement, angiolymphatic, venous, or perineural invasion, and surgical margin were routinely reported in the pathologic reports at our hospital. After curative-intent surgery, microscopically (R1) or marcroscopically (R2) residual tumor was noted in 68 patients, while 71 patients did not (R0). The survival data were obtained from the official death reports issued by the government.
Statistical analysis
To determine predictive factor for margin positive resection (R1 or R2) on pre-operative CT, a chi-square test was used for univariable analysis (likelihood of BD involvement from intrapancreatic CBD to bilateral second-order branches, peritumoral fat infiltration, the relation between tumor and vessels, and LN involvement). The parameters that proved to be significant on the univariable analysis (tail p-values less than 0.1) were then subsequently tested with the multivariable using logistic regression. The survival rate during the follow-up period was reported with a 95% confidence interval using the Kaplan-Meier method. After univariable analysis of each parameter (pre-operative image findings, laboratory results, and extent of surgery) using a log-rank test, the parameters that proved to be significant (tail p-values less than 0.1) were then subsequently tested with the multivariable analysis using Cox proportional hazard model. Interobserver agreement was assessed using weighted κ statistics for noncontinuous scale and intraclass correlation coefficient (ICC) for continuous scale. The strength of agreement was evaluated as follows: a κ value and ICC value of less than 0.20 indicated poor agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderated agreement; 0.61-0.80, good agreement; and 0.81-1.0, excellent agreement. The analysis were performed using commercial statistical softwares (SPSS version 23, IBM Corporation, Armonk, NY or MedCalc ver. 16.4, MedCalc software, Marikerkem Belgium). For all statistical analysis, P value less than 0.05 were considered statistically significant.
Results
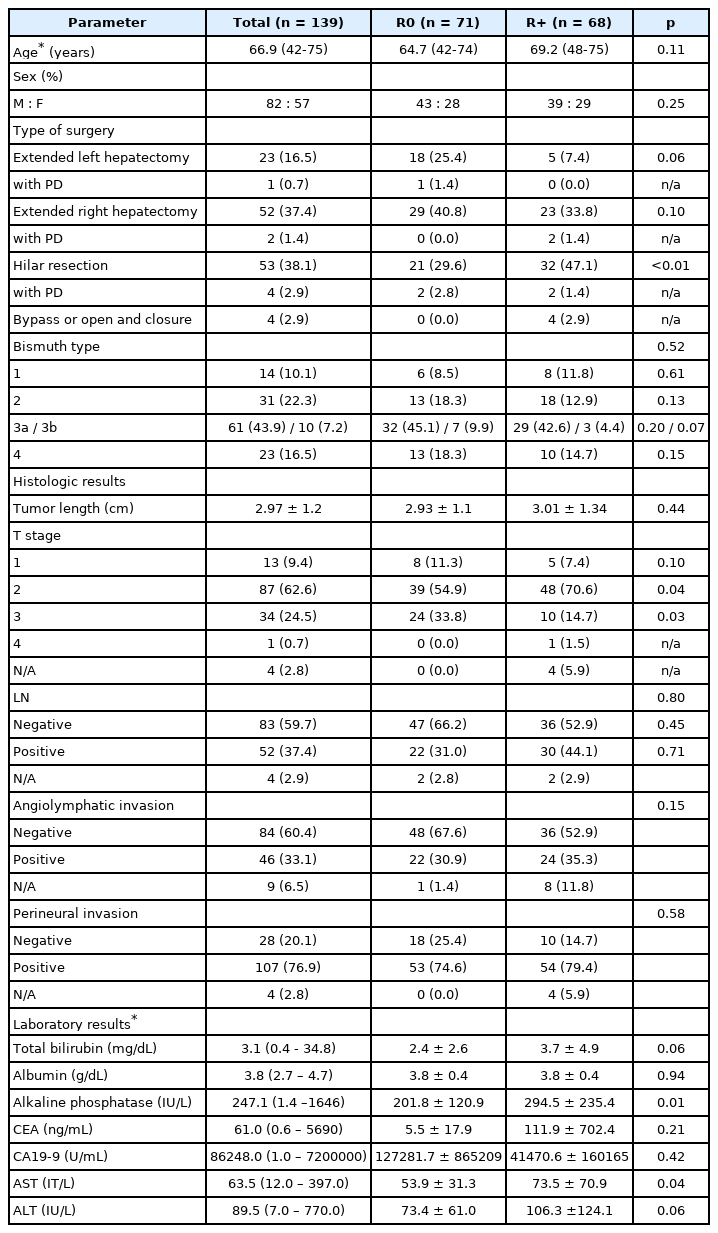
After curative-intent surgery, microscopically (R1, n = 44) or marcroscopically residual tumor (R2, n = 24) were noted in 68 patients. Of 68 patients, 45 (66.2%) were involving proximal margin, 29 (42.6%) were distal margin, 10 (14.7%) were radial margin, and 2 (2.9%) were presenting seeding nodules. Eighteen patients (12.9%) were showing surgical margin positive at more than two site. T stage of PHC were as follows: T1, n = 13 (9.4%); T2, n = 87 (62.6%); T3, n = 34 (24.5%); T4, n = 1 (0.7%). Four patients (2.8%) were not applicable for T stage due to receive bypass or open and closure surgery. Fifty-two patents (37.4%) presented pathologically proven LN metastasis, and there is no significant difference between R0 and R+ group. More patient (n = 26, 38.2%) underwent hilar resection in R+ group (p < 0.01) than R0 group. Thirty-eight patients (28.5%) presented anatomic variation of bile duct: trifurcation (n = 13), right posterior bile duct drains to common hepatic duct (n=13), and right posterior bile duct drains to left hepatic duct (n = 12). There is no variation showing and right posterior bile duct drains to cystic duct. Table 1 summarized patient's demography, clinical and histopathological features.
Important CT findings for complete resection of perihilar cancer
Table 2 summarized important CT findings according to the resection margin status in perihilar cancer. By comparing histopathologic results, the AUC value of mid CBD involvement on pre-operative CT was 0.71 (cutoff score ≥ 4; sensitivity, 87.2%; specificity of 55.5%) and intrapancreatic CBD involvement was 0.72 (cutoff score ≥ 4; sensitivity, 60.0%; specificity of 91.6%). On univariable analysis, mid-CBD (score ≥ 4) or intrapancreatic CBD (score ≥ 4) involvement on pre-operative CT were significant factors for margin positive resection (p = 0.045 and 0.045, respectively). None of the vessel involvement, peritumoral fat infiltration, or lymphadenopathy on pre-operative CT were presented significant differences in resectability. On multivariable analysis, mid CBD involvement (score ≥ 4) on pre-operative CT (odds ratio = 3.09, 95% CI 1.44-6.67, p = 0.004) remained significant factor for margin positive resection (Table 2, Fig. 2). The mid CBD involvement (score ≥ 4) on pre-operative CT predicted 92.9% (26/28) of distal margin positive resection (p = 0.002), but did not associated with proximal margin (p = 0.254) or radial margin positive resection (p = 0.435).
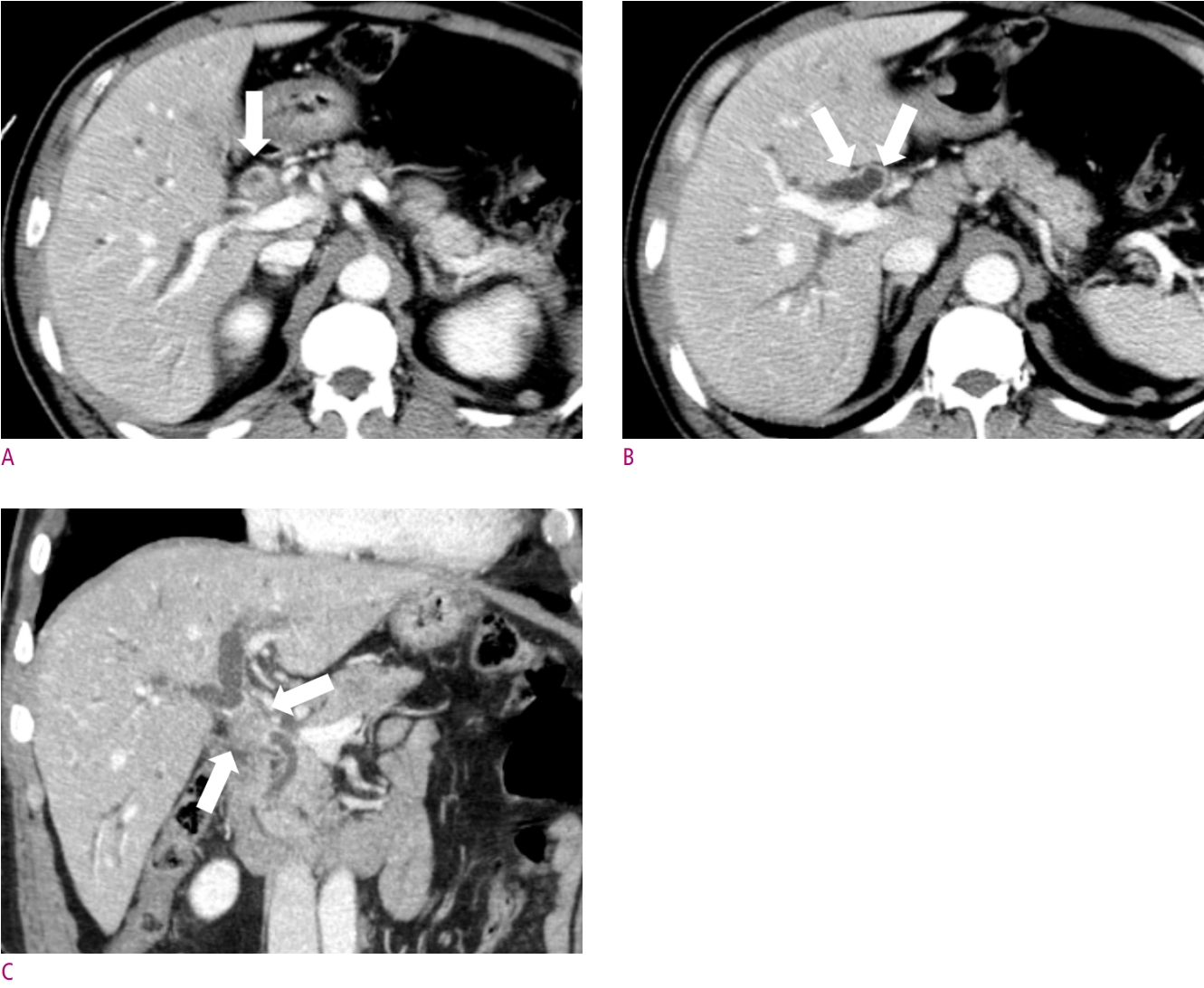
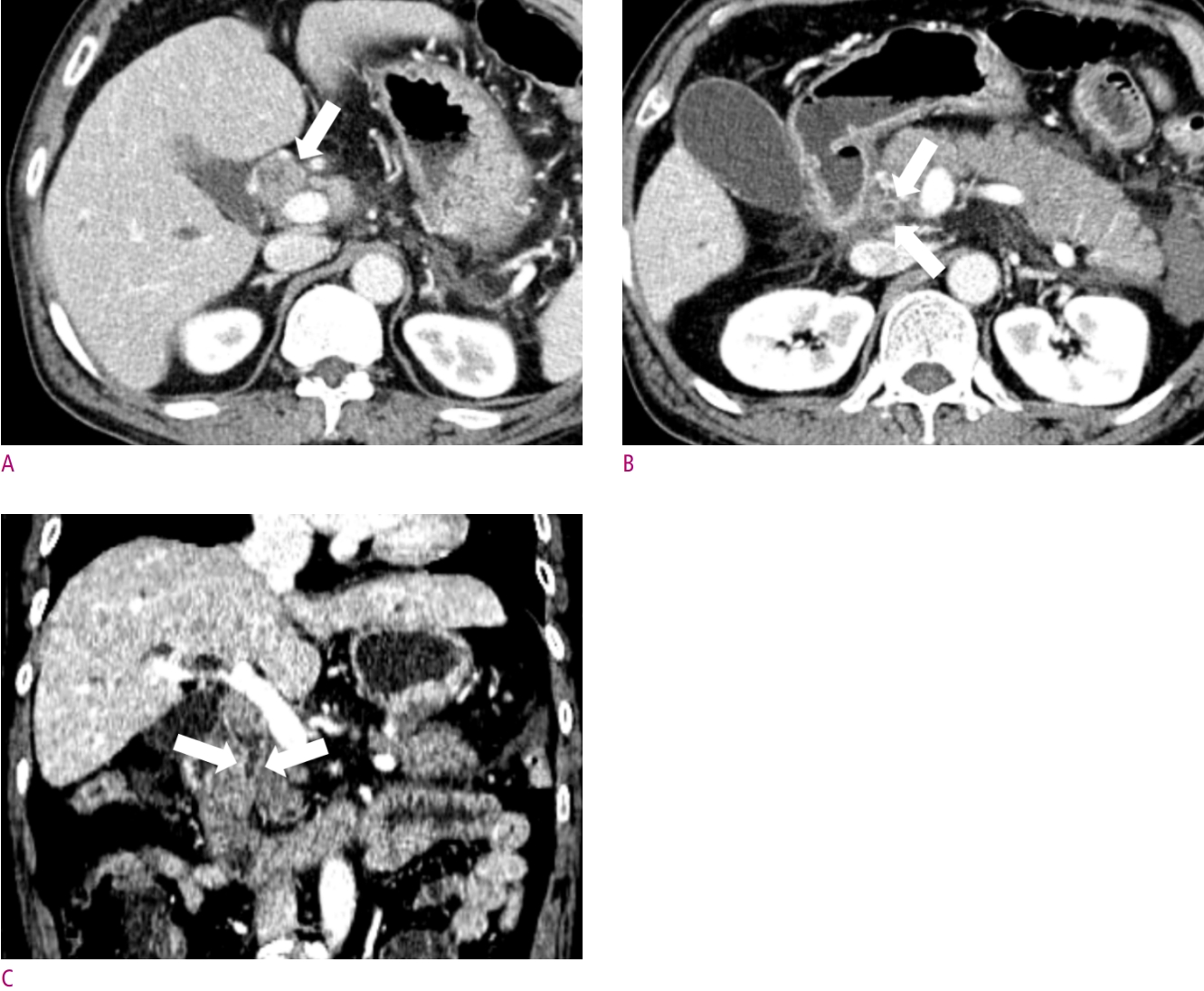
A 63-year-old female patient with jaundice.
(A) Concentric enhancing wall thickening was noted at proximal common bile duct (CBD) (arrow). (B) This lesion is proximally extended to proximal right and left hepatic duct (arrows), and (C) distally extended to mid CBD (arrows), which suggestive of Bismuth type II perihilar cancer. After segmental duct excision, distal resection margin was involved by adenocarcinoma on pathologic report.
In assessment of tumor extent, good to excellent interobserver agreement were found: intrapancreatic CBD (ICC = 0.83), mid-CBD (ICC = 0.73), first order of IHD (κ = 0.63) and second order of IHD (κ = 0.67) involvement. Regarding vessel relation assessment (κ = 0.32, 0.52 and 0.74 for RPV, LPV and MPV, κ = 0.35 and 0.52 for RHA and LHA, respectively) and peritumoral fat infiltration (ICC = 0.47), fair to good agreement were found.
Important clinical and CT findings for overall survival after curative intent surgery
Of 139 patients, 112 patients (80.6 %) were deceased owing to the following cause: progression of PHC (n = 101), pneumonia (n = 5), progression of other malignancies (n = 3), and sepsis (n = 3). The mean follow up time was 37.8 ± 34 months (95% confidence interval [CI], 19.2 – 31.4 months). The median survival was 25.3 ± 3.12 months (interquartile range, 11.5 – 55.9 months). The mean survival was 24.8 ± 19.5 months, and mean follow-up period for survivor was 92.0 ± 27.8 months.
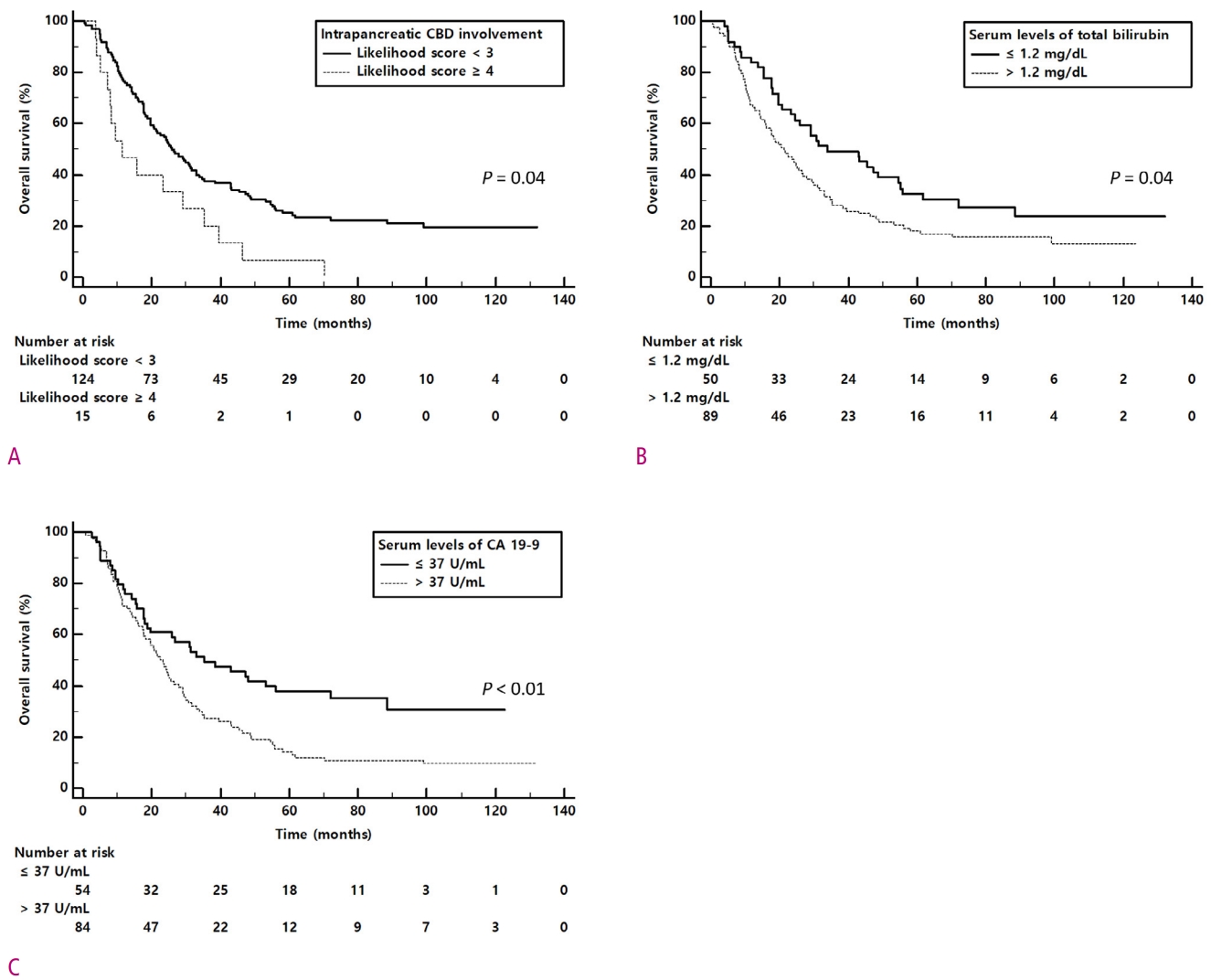
Table 3 summarized important clinical and CT findings for overall survival after curative intent surgery in perihilar cancer. In univariable analysis, elevated level of total bilirubin (p = 0.03) and CA 19-9 (p = 0.02), intrapancreatic CBD (p < 0.01) and second order branches of IHD involvement (p = 0.07) on pre-operative CT were significant risk factor for survival. On multivariable analysis, increased level of total bilirubin (> 1.2 mg/dL , hazard ratio [HR] = 1.53, p = 0.04), CA 19-9 (> 37 U/mL, HR = 1.75, p < 0.01), and intrapancreatic CBD involvement (score ≥ 4, HR = 1.81, p < 0.01) on pre-operative CT were the significant predictor for poor overall survival (Figs. 3 and 4).

Summary of important clinical and CT findings for overall survival after surgery in perihilar cancer
A 69-year-old male patient with jaundice.
(A) Intraductal growing lesions are noted in proximal common bile duct (CBD) (arrow). (B) This lesion is proximally extended to proximal right and left hepatic duct (arrows), and (C) distally to intrapancreatic CBD (arrows), which suggestive of Bismuth type II perihilar cancer. After pylorus-preserving pancreaticoduodenectomy, surgical margin was negative. However, the patients was deceased 125 days after surgery due to surgical complication.
Discussion
In our study, we found that mid-CBD involvement in pre-operative CT imaging was a significant factor for margin positive resection. In survival analysis, intrapancreatic CBD involvement in pre-operative CT was a significant factor for poor survival (HR = 1.81, p < 0.01). Even though there have been many studies that stated a significant relationship between vascular involvement and resectability [28-30], there are limited studies for the relationship between the distal extent of tumor and resectability. Also, it is notable that high interobserver agreements in evaluating intrapancreatic CBD (ICC = 0.83) and mid-CBD (ICC = 0.73) involvement, which are crucial for consistent pre-operative planning.
We also found that the diagnostic ability of pre-operative CT for mid-CBD involvement was 0.71 (AUC value) with 87.2% sensitivity and 55.5% specificity. In addition, a likelihood score of 4 or 5 for mid-CBD involvement on pre-operative CT predicts 92.9% of distal margin positive resection. These results are easily explainable as when the tumor involves distally, the surgeon have to decide whether undergo pancreaticoduodenectomy together or not. However, combining operation of pancreaticoduodenectomy and hilar resection, the one of major surgery, may not easy to determine due to increased morbidity and mortality of operation. Thus, only some patients are allowed to undergo combining pancreaticoduodenectomy.
For survival analysis, intrapancreatic CBD involvement was the independent predictor for poor survival. Our hypothesis of this result is the intrapancreatic CBD involvement in pre-operative CT relates to high tumor burden. Another possible hypothesis is that broad surgery extent such as pancreaticoduodenectomy when intrapancreatic CBD involvement is suspected, increase mortality and morbidity of the surgery. However, in our study, extended surgery is not a significant factor for overall survival.
The diagnostic ability of pre-operative CT for intrapancreatic CBD involvement was 0.72 (AUC value) with 60.0% sensitivity and 91.6% specificity. These results were in good agreement with the results of a previous study showing the 82% accuracy to assess the distal border of the tumor using pre-operative CT examination [31]. In addition, the interobserver agreements of intrapancreatic CBD (ICC = 0.83) and mid-CBD (ICC = 0.73) on pre-operative CT were high which are crucial for consistent pre-operative planning. Thus, we cautiously suggest that evaluating the distal extent of perihilar cholangiocarcinoma using pre-operative CT is feasible for resectability evaluation and patient's survival.
In our study, elevated serum levels of CA 19-9 (> 37U/mL p < 0.01) and total bilirubin (> 1.2 mg/dL, p = 0.04) were the independent prognostic factors for poor overall survival. Although CA 19-9 is a well known diagnostic marker for cholangiocarcinoma, whether it can be used as a prognostic marker has not been studied. Recently, however, a few studies have been reported CA 19-9 as a potential prognostic marker. Chaiteerakij et al. [32] proposed the CA 19-9 level of 1000 U/mL or more as the variables in perihilar cancer staging system. Oguro et al. [33] reported that CA 19-9 level of 64 U/mL or more was the significant factor for poor overall survival with hazard ratio of 1.9. Also in our study revealed that the elevated serum level of total bilirubin in pre-operative period was an independent predictor for poor survival. Our results are well in line with a previous study of Koerkamp et al. [34] which had reported the total bilirubin level of 2 mg/dL or more was the significant factor for poor 5-year recurrence free survival. One of plausible explanation of this results is that the pre-operative level of total bilirubin may reflect the severity of disease, even with the appropriate intervention to lower bilirubin level.
In our study, vascular invasion is not a significant factor for resectability assessment. This results are not consistent with previous studies stated vascular invasion as a poor prognostic factor [4, 29]. The discrepancy is explainable as our study population are limited for those who are possible curative-intent surgery and well-known contraindicated cases such as contralateral artery or portal vein invasion [29] were excluded. When the vessel involvement is in the operable ranges for curative intent surgery, vascular involvement did not influence resectability and patient's survival.
There are several limitations in our study. First, owing to the nature of single center based retrospective study, selection bias was not avoidable. Second, their resectability might be influenced by surgeon's propensity. However, as one of big centers undergoing operation for perihilar cholangiocarcinoma about 50 cases per year, our hospital have followed standard treatment strategy based on multi-disciplinary approach. Thus, the effect of surgeon's propensity would be minimized.
In conclusion, mid-CBD involvement on pre-operative CT is significant factor for margin positive resection on curative intent surgery and intrapancreatic CBD involvement is significant factor for poor survival. Therefore, distal longitudinal extent of perihilar cancer on pre-operative CT would be an important factor for margin positive resection and poor survival on curative intent surgery.